Gold catalysts
Gold catalysis is one of the most dynamic research fields in catalysis. In the recent years we successfully developed new preparation methods for gold catalysts and new application fields for their use.
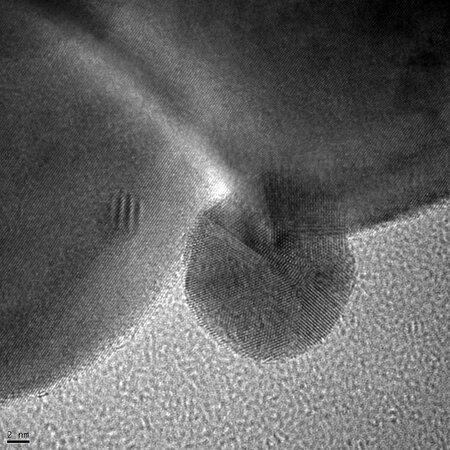
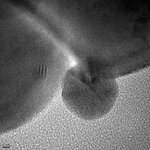
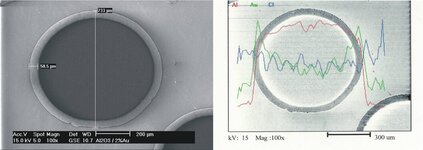
Until the late 1980s Gold catalysts were considered unsuitable for catalytic reactions because of their low activity. It was found that the activity of gold catalysts depends strongly on the presence of small gold nanoparticles. With the development of new preparation methods, e.g. deposition-precipitation, highly active supported gold catalysts with gold nanoparticles were made available. Since then gold catalysis is one of the most dynamic research fields in catalysis.
We applied gold catalysts in different reactions including the oxidation of carbohydrates to sugar acids. The newly developed supported gold catalysts were used to convert e.g. glucose, maltose and lactose to gluconic acid, maltobionic acid and lactobionic acid in virtually 100% product yields. Additionally to the high activity and selectivity the gold catalysts exhibited excellent long term stability. Our research on catalyst development and process optimization lead to the upscaling of the process into demonstration scale.
A new preparation method for gold catalyst based on the incipient-wetness method (IW method) was also developed. The IW method formerly was in general considered as unsuitable for the preparation of gold catalysts. We modified the IW method regarding the impregnation procedure and the thermal treatment which resulted in highly active catalysts. This development is also of industrial importance because impregnation methods in general are simple, inexpensive and easy to scale-up.
Our research in the field of gold catalysis involved furthermore the synthesis of bimetallic supported catalysts with platinum as a secondary metal. These catalysts were successfully applied in the oxidation of fatty alcohol ethoxylates to their corresponding carboxylic acids which are used as surfactants and detergents. Higher activities and yields compared to catalysts in the literature could be achieved. More important, higher ether carboxylic acid yields than in the current industrial production process can be achieved.
Bimetallic gold platinum catalysts were additionally used for the oxidation of sorbose to 2-keto-L-gulonic acid. The reaction has the potential to facilitate the industrial production of ascorbic acid (vitamin C).